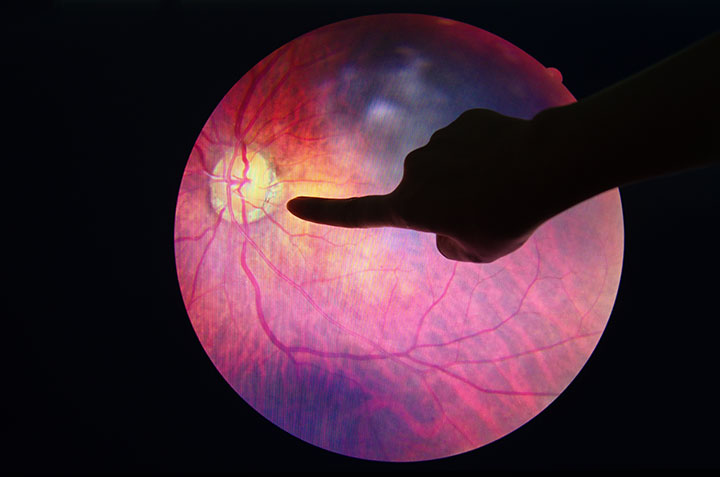
On April 11, the U.S. Food and Drug Administration (FDA) cleared IDx-DR, the first medical device to use artificial intelligence (AI) to detect greater-than-mild level of diabetic retinopathy. IDx-DR is a software program that uses an AI algorithm to analyze images of the eye taken with a retinal camera. The digital images are uploaded to a cloud server on which AI software is installed. If the images are of sufficient quality, the software provides the provider with one of two results:
- more than mild diabetic retinopathy detected: refer to an eye care professional
- negative for more than mild diabetic retinopathy; rescreen in 12 months.
If a positive result is detected, patients should see an eye care provider for further diagnostic evaluation and possible treatment as soon as possible.
You can learn more about this device at FDA.gov.