Understanding these rapidly evolving treatments will help you advocate for patients and manage side effects.
By Beth Boseki, MSN, RN, OCN, CCRP; Lauren FInaldi, BSN, RN, OCN; Samantha Siegel, MSN, APN-C, OCN
Takeaways:
- Immunotherapy is steadily becoming the standard of care for cancer patients.
- Immunotherapy hand, works with the body’s immune system by helping T cells to better distinguish cancer cells from healthy cells and shrink or kill them.
- The side effects of T-cell stimulation are inflammatory and include rash, diarrhea, liver inflammation, and hypothyroidism.
Immunotherapy is steadily becoming the standard of care for cancer patients. The rapidly advancing science behind immunotherapy offers oncology patients treatment options with durable responses and potentially less toxicity. Immunotherapy encompasses several treatment modalities, including oncolytic viruses, checkpoint inhibitors, and chimeric antigen receptor (CAR) T-cell therapy. All of these options work with the body’s own immune system to identify abnormal cancer cells that can be destroyed while healthy normal cells are unharmed. Understanding these options helps nurses better care for patients, including providing education.
How immunotherapy works
The immune system is the body’s defense against infectious organisms and other invaders. It’s made up of a network of cells (including T cells and B cells), tissues, and organs working together to protect the body by attacking organisms and substances that in-vade the body and cause disease.
Cancer cells, however, are able to evade cell death and escape immune recognition and subsequent destruction. They do this by developing multiple resistance mechanisms, including local immune evasion, induction of tolerance (which results in unresponsiveness of the immune system), and systemic disruption of T-cell signaling.
Chemotherapy destroys rapidly dividing cells, such as cancer cells, but it also attacks other rapidly dividing healthy cells, such as blood and mucosal cells. This is what causes side effects such as alopecia, mouth sores, nausea, vomiting, diarrhea, and decreased blood cell counts.
Immunotherapy, on the other hand, works with the body’s immune system by helping T cells to better distinguish cancer cells from healthy cells and shrink or kill them. The side effects of T-cell stimulation are inflammatory and include rash, diarrhea, liver inflammation, and hypothyroidism.
In this article, we’ll review three immunotherapy treatment options oncolytic viral immunotherapy, checkpoint inhibitors, and CAR T-cell therapy—including their indications and nursing implications.
Oncolytic viral immunotherapy
Vaccines, which take advantage of the body’s defensive cell-mediated immunity response, have been developed as prophylactic treatment to prevent cancer-causing viruses—such as human papillomavirus and hepatitis B—but their use in treating malignancies is still evolving.
Cancer treatment vaccines work by stimulating T cells (usually CD8 positive or helper CD4 positive cells) and enabling them to recognize and act against specific cancer types or by inducing the production of antibodies that bind to molecules on the surface of cancer cells. Cancer vaccines are composed of weakened or killed cancer cells that carry a specific cancer antigen or an immune cell that’s modified to act as an antigen. These cells can be autologous (come directly from the patient) or allogenic (from another person).
Indications
In 2010, the U.S. Food and Drug Administration (FDA) approved the use of sipuleucel-T for treating resistant metastatic prostate cancer. The vaccine is created by drawing the patient’s blood and then isolating certain immune cells identified within it. That blood is then fused with an immune-cell stimulator in the laboratory and infused back into the patient.
Another FDA-approved oncolytic virus is talimogene laherparepveca genetically modified version of the herpes simplex virus that causes cold sores. The vaccine is administered directly into the melanoma lesion, which causes the cells to rupture and die. Several oncolytic viral agents are currently in clinical trials.
Nursing implications
The most common side effects from cancer vaccine therapy are inflammatory responses at the injection site, such as erythema, pain, edema, heat, rash, and pruritus. Other potential adverse events include flulike symptoms, low-grade fever, nausea, vomiting, myalgia, headache, and fatigue. Because vaccines produce immune-mediated responses, the risk of a hypersensitivity reaction exists and should be considered a medical emergency.
While administering these vaccines, adhere to hazardous drug safe-handling principles. Personal protective equipment, including gloves and gowns, is required. And make sure you’re familiar with your organization’s guidelines for administering immunotherapy.
Checkpoint Inhibitors
Checkpoint inhibitors are among the newest agents used in cancer therapy. In a healthy human, the immune system has the innate ability to identify foreign cells and attack them while safeguarding normal tissue. Cancer cells take advantage of abnormalities that cause decreased expression of checkpoint proteins and manipulate the body’s natural defensive response so they can continue to proliferate.
Recent literature discusses the concept of microsatellite instability (MSI). Cancer cells in tumors with an MSI-high (MSI-H) status have a greater than normal number of genetic markers called microsatellites, which are short, repeated sequences of DNA that may relate to defects in the cells’ ability to correct mistakes that take place when DNA is copied. This defect is most frequently found in colorectal cancer, but other types of GI cancer and endometrial cancers may have MSI-H. This can put the patient at a higher risk of other cancers and also may be relevant for genetic testing of immediate family members.
Preliminary data suggest that patients with MSI-H status cancers (which means they have high mutational tumor burden) are more likely to respond to checkpoint inhibitors. MSI-H tumors also have been shown to respond to anti-programmed cell death protein (PD-1) checkpoint inhibitors.
Immune checkpoint inhibitors approved by the FDA include atezolizumab, avelumabm, durvalumab, ipilimumab, nivolumab, and pembrolizumab. These agents use different mechanisms to inhibit different checkpoints, although they’re all T-cell suppressors (T-cell agonists). For example, ipilimumab is a monoclonal antibody that inhibits cytotoxic T lymphocytes. It binds to cytotoxic lymphocyte-associated molecule-4 (CTLA-4) proteins CD80 and CD86 ligands, blocking the CTLA-4 pathway and enhancing T-cell proliferation and antitumor effects. Nivolumab and pembrolizumab work on PD-1 and atezolizumab targets the programmed death ligand 1 (PD-L1). When PD-1 is bound to the PD-L1 ligand, it produces an immune response and antitumor effects. (See Making a block.)
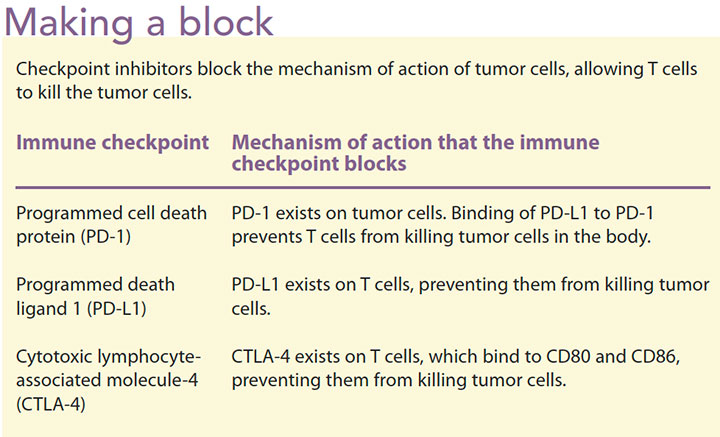
Indications
Current indications for checkpoint inhibitors include melanoma, nonsmall cell lung cancer, renal and bladder cancer, Hodgkin’s lymphoma, and head and neck cancer. The FDA indications for these agents are rapidly evolving, and new indications continue to be added to the National Comprehensive Cancer Network Guidelines and Compendia. For patients who have cancers that aren’t currently FDA approved for treatment with checkpoint inhibitors, individual drug companies may offer them via compassionate use. However, their use is up to the treating oncologist’s discretion.
Nursing implications
Unlike conventional chemotherapy, checkpoint inhibitors stimulate T cells, which elicits an inflammatory reaction in healthy organs. This, in turn, can cause inflammatory side effects, particularly in the GI tract (for example, colitis), endocrine glands (for example, pancreatitis, thyroid dysfunction, adrenal insufficiency), skin (for example, dermatitis, pruritis), and liver (for example, hepatitis).
Many of these toxicities may be mild, requiring only a short course of steroids, but severe toxicity may require emergent treatment, including hospitalization and consultation with a specialist. The American Society of Clinical Oncology has released a guideline with recommendations for managing toxicities from immune checkpoint inhibitors based on grade of toxicity.
Side effects can occur at any time throughout treatment, and because patients typically are on these therapies for prolonged periods, ongoing monitoring is vital. Early detection (through patient assessment and laboratory testing) and prompt treatment are critical to managing checkpoint inhibitor toxicities.
Teach patients and family members about the potential toxicities, and stress the importance of notifying all healthcare providers that the patients are being treated with checkpoint inhibitors. Patients should carry a wallet card that lists the agents they are receiving. In addition, instruct patients to contact their oncology team if they experience any side effects.
Chimeric antigen receptor T-cell therapy
The revolutionary CAR T-cell therapy, which targets CD19 antigen through a specified immune response, has been used to treat patients with relapsed acute lymphoblastic leukemia (ALL).
In a healthy body, both T and B cells are active in maintaining a healthy immune system. When CAR T-cell therapy is administered, the immune antigens identify B cells that express CD19 and activate an immune response. However, the therapy can’t yet differentiate between B cells infected with malignancy and healthy B cell antigen. This results in death of all B cells, which can lead to toxicity and side effects.
Memorial Sloan Kettering Cancer Center, University of Pennsylvania, National Cancer Institute, and Fred Hutchinson Cancer Research Center enrolled 158 subjects of varying ages with B-cell ALL in their CAR T-cell therapy trials. The results revealed 134 complete responses (disappearance of all areas of disease), an 85% response rate. This is a significant tumor response, especially since ALL in pediatric patients is considered incurable. About 33% of the 134 subjects at these cancer centers have reported relapses. Research is ongoing to discover why the relapses occurred and how those who are still in remission have maintained their health.
Indications
CAR T-cell therapy has significant risk, but the treatment response rate is impressive, so the FDA granted approvals for adult and pediatric patients with relapsed refractory B-cell ALL, as well as patients with relapsed refractory diffuse large B-cell lymphoma.
Nursing implications
CAR T-cell therapy is a multistep process. (See CAR T-cell process.) Before initiating the CAR T-cell therapy, a certified nurse must perform leukapheresis to harvest white blood cells. After harvesting the cells, the process of engineering the CART-19 can take up to 3 weeks. In the meantime, patients begin lymphodepleting chemotherapy (such as cyclophosphamide and fludarabine) to control the disease.
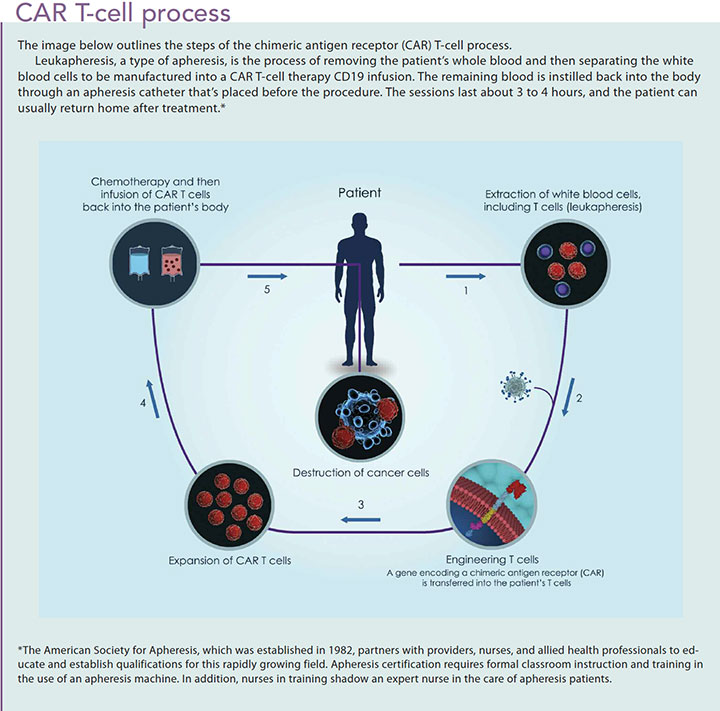
About 1 week after chemotherapy administration, the CAR T-cell therapy is introduced. Patients are premedicated with acetaminophen and diphenhydramine to prevent or lessen potential toxicities. Some facilities require an overnight hospital stay to monitor patients, especially if they acquire a fever as an immune response. Continuous monitoring is required to ensure safety precautions are met, so it’s not surprising that this therapy is administered only at large medical centers that participate in active oncology research or have a bone marrow transplantation unit. A lower nurseto-patient ratio is vital to ensure adequate monitoring.
The most common toxicities seen with CAR T-cell therapy are cytokine release syndrome (CRS), neurologic symptoms, graft-versus-host disease, and tumor lysis syndrome (TLS). Both CRS and TLS are oncologic emergencies, but they present differently. (See CAR T-cell therapy toxicities.) Although nursing interventions for immunologic oncologic emergencies are similar in the care of patients receiving CAR T-cell therapy, vital signs should be taken every 4 hours and labs (including electrolytes) should be drawn at least once a day.

The future of CAR T-cell therapy is evolving as long-term results, side effects, and efficacy have not yet been determined. Researchers also are developing a new approach to this therapy by determining if harvesting CD19 antibodies from healthy donors would be more beneficial than the current process of harvesting from the patient. Research also is being conducted in solid tumor studies to determine the efficacy of engineering a “super T cell” to override the immune suppressor genes in advanced solid tumors.
Evolving treatment
The advent of immunotherapy and targeted/novel agents is changing oncology care. Immunotherapy has given hope and longer survival benefits to oncology patients. The rapid evolution of these treatments means that nurses and other healthcare professionals must stay abreast of research updates so they can communicate treatment optionsincluding indications, toxicities, and self-care—to their patients.
Beth Boseki is a nurse practitioner at Memorial Sloan Kettering Symptom Triage Clinic in Basking Ridge, New Jersey. Lauren Finaldi is the phase I clinical research nurse coordinator at The John Theurer Cancer Center at Hackensack Meridian Health in Hackensack, New Jersey. Samantha Siegel is a nurse practitioner at Memorial Sloan Kettering Cancer Center in Monmouth, New Jersey.
Selected references
Bayer V, Amaya B, Baniewicz D, Callahan C, Marsh L, McCoy AS. Cancer immunotherapy: An evidence-based overview and implications for practice. Clin J Oncol Nurs. 2017; 21(2 Suppl):13-21.
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;14(4):247-49.
Callahan C, Baniewicz D, Ely B. CAR T-cell therapy: Pediatric patients with relapsed and refractory acute lymphoblastic leukemia. Clin J Oncol Nurs.2017;21(2 Suppl):22-8.
Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol.2016;2(10):1346-53.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med.2018;378(2):158-68.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561-84.
National Cancer Institute. Cancer vaccines. 2015.
National Cancer Institute. Immunotherapy to treat cancer. 2018.
National Cancer Institute. NCI dictionary of cancer terms: Immune checkpoint inhibitor.
National Cancer Institute. NCI dictionary of cancer terms: MSI-H cancer.
Peterson JJ, Steele-Moses SK. Update on new therapies with immune checkpoint inhibitors. Clin J Oncol Nurs.2016;20(4):405-10.
Smith L, Venella K. Cytokine release syndrome: Inpatient care for side effects of CAR T-cell therapy. Clin J Oncol Nurs.2017;21(2 Suppl):29-34.
Wiley K, LeFebvre KB, Wall L, et al. Immunotherapy administration: Oncology Nursing Society recommendations. Clin J Oncol Nurs. 2017;21(2 Suppl):5-7.
ant7-CE Immunotherapy-618
















2 Comments.
information was good about how immunotherapy effects the body. Side effects and patient education. I feel is a key to immunotherapy being successful. Mary Ann Ford
very informative feel I could identify side effects. plus need educated patients fo what they should watch for and seek medical attention.