In a new report, The National Toxicology Program (NTP) expressed ”serious concern” that diethylhexyl-phthalate (DEHP), a chemical used in medical devices, may affect male reproductive tract development and harm infant males undergoing intensive medical treatments. This reaffirms similar concerns expressed in a 2000 NTP report.
The recent report also suggests that male offspring of pregnant and breastfeeding women may be exposed to DEHP as a result of their mothers undergoing medical procedures. In 2002, the Food and Drug Administration (FDA) also expressed concern, but extended this concern to include the male fetus, male neonate, and peripubertal male as being at high risk from exposure to DEHP in medical settings. These governmental warnings about phthalates, however, may only be the tip of a hazardous chemical iceberg.
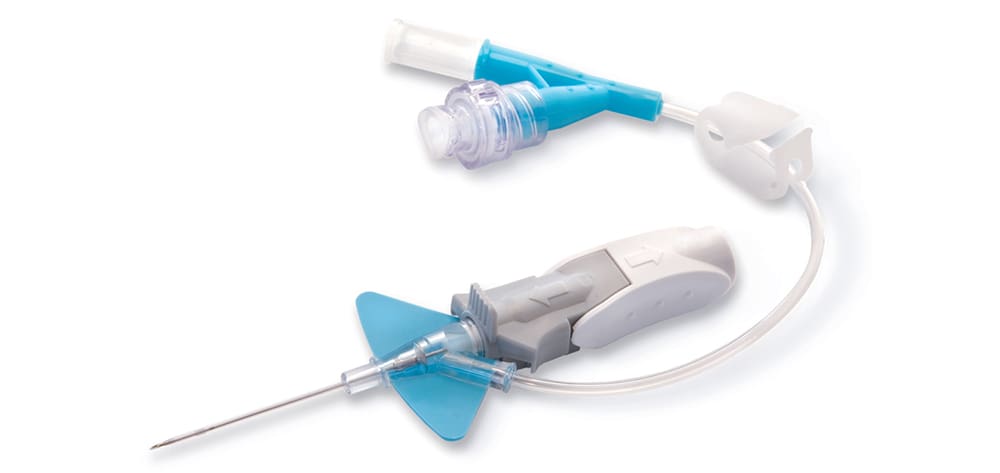
DEHP is used in plastics (for example, I.V. bags and tubing) to make them pliable. Unfortunately, it can leach out of the plastic and into I.V. fluids and enteral feeding products that enter patients. It is also found in a variety of other medical equipment that comes into direct contact with patients. Other phthalates with somewhat variable characteristics are useful in such products as automobile parts, tools, toys, food packaging, paints, glues, perfumes, personal-care products, lubricants, and wood finishes.
Phthalates are ubiquitous in the environment. Humans are exposed to these chemicals through the air we breathe, food we eat, and water we drink. Human studies have found several phthalates present in the general U.S. population.
Studies reveal phthalates’ impact
Initial concern stemmed from the impact of some phthalates on laboratory animals. Particularly alarming are studies that showed “demasculinized” effects in male offspring, such as cryptorchidism, hypospadias, low sperm counts, and reduced fertility.
A disturbing phenomenon, possibly reflected in the animal data, is the apparent decline in human male reproductive health worldwide. Researchers have noted an increase in testicular cancer, hypospadias, and cryptorchidism, and a decrease in sperm concentrations and quality in some areas. It has been proposed that these symptoms are part of a syndrome caused by prenatal exposure to endocrine-disrupting environmental chemicals. Phthalates, along with other chemicals to which humans are exposed, are endocrine disruptors. Several phthalates, including DEHP, reduce testosterone synthesis, which is likely to contribute to the observed impact in laboratory studies.
While the animal studies prove that a risk exists, a clear connection between chemical exposure and effect on human health is more difficult to establish than in animal laboratory studies. One recent study found an association between altered genital measurements in infant boys and their prenatal exposure to phthalates as measured in their mothers’ urine. Other studies have found decreased semen quality associated with phthalate exposure in grown men. Further studies are needed.
Unfortunately, even the most well-designed human studies do not take into account the effects of multiple chemical exposures. It is important to consider that infant boys, apparently the population most vulnerable to this particular chemical group, face exposure to many other phthalates during medical treatments. Even the male fetus is exposed to phthalates. One study found that women of child-bearing age had the highest level of phthalate metabolites in their urine, possibly due to their use of such personal-care products as cosmetics and perfumes, thereby potentially exposing an unborn fetus. Another source of infant exposure may come after birth, because phthalates have been found in breast milk.
The infant and young child may be further exposed to phthalates through baby products. One study found that elevated phthalate metabolites in young children coincided with increased use of these products; for example, plastic baby toys, on which infants commonly suck. Currently, we do not know the implications of these multiple exposures, although animal testing shows that some phthalates are additive in their impact.
What can nurses do? Although the FDA recommends using non-DEHP-containing medical equipment, it does not require labeling for DEHP-containing equipment, thus making informed decision making difficult. One thing nurses can do is write to the FDA and demand that such labeling be required: Commissioner, Food and Drug Administration, Parklawn Building, Room 1471, 5600 Fishers Lane, Rockville, MD 20877.
For more information on ridding the workplace of DEHP, go to www.noharm.org/us/pvcDehp/issue.
Marian Condon, MS, RN, is Senior Staff Specialist for ANA’s Center for Occupational and Environmental Health.