Using the right securement method can improve outcomes and reduce costs.
Takeaways:
- Failure of vascular access devices (VADs) can be attributed to inserter characteristics, patient-related factors, anatomic placement, and healthcare facility adherence to international best practices and infection prevention guidelines.
- Current VAD securement solutions can be grouped into five categories: sutures, adhesive securement devices, subcutaneous securement devices, tissue adhesives/“superglue” (cyanoacrylate), and integrated securement solutions.
 By Timothy R. Spencer, DipAppSc, BHSc, RN, APRN, ICCert, VA-BC™
By Timothy R. Spencer, DipAppSc, BHSc, RN, APRN, ICCert, VA-BC™
Many clinicians consider the placement of peripheral and central vascular access devices (VADs) to be a routine and mundane interventional procedure, yet these devices are associated with significant morbidity and mortality. Localized complications of peripheral intravascular (intravenous) catheters (PIVCs), such as phlebitis and infiltration, are underreported,but they’re known to contribute to PIVC failure. (See Understanding the cost of PIVC failure.) This underreporting has made it difficult to identify contributing factors for failure, which may include inserter characteristics, patient-related factors, and anatomic placement, as well as healthcare facility adherence to international best practices and infection prevention guidelines.
Central VAD (CVAD)failure also causes significant problems. For example, a systematic review by Ullman and colleagues described the rate of CVAD failure and complications across CVAD types in pediatrics within the international healthcare community. Applying the rate of failure described in their study, 5,457 pediatric and neonatal CVADs in U.S. hospitals failed before treatment completion in 1 year alone. These failures place a massive economic and physical burden on the U.S. healthcare system, patients, and families.
Many peripheral and central VAD complications can be avoided with clinician attention to technique, appropriate securement during device selection and placement, and up-to-date organizational guidelines, policies, and procedures. This article focuses on securement.
Importance of securement—and the right dressing
After any VAD is inserted, it must be appropriately secured to reduce complications. Fortunately, the last 2 decades have seen advancements in securement techniques. Nurses, providers, and other clinicians need to apply evidence-based best practices when selecting securement options to help prevent VAD complications, including catheter-related infection and thrombosis.
The dressing protects the insertion site, and the securement method directly influences dressing management. Movement frequently disrupts dressing adhesion, and dressing removal is a pivotal procedure that can affect VAD stability. Small movements, whether in and out or side to side, can increase the potential for securement problems.
Global clinical practice guidelines state that PIVC dressings should be clean, dry, and intact, and that they should be well secured. However, 21% to 71% of PIVC dressings are soiled, moist, loose, or inadequately secured, according to a study by Rickard and colleagues.
Securement solutions
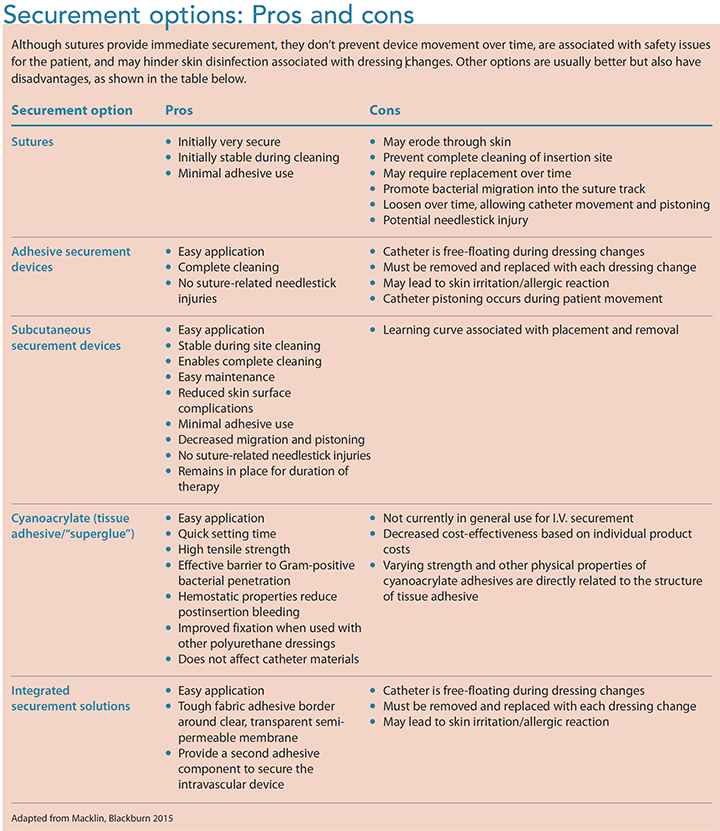
Current VAD securement solutions can be grouped into five categories: sutures, adhesive securement devices, subcutaneous securement devices, tissue adhesives/ “superglue” (cyanoacrylate), and integrated securement solutions. (See Securement options: Pros and cons.) Adhesive and subcutaneous securement devices are grouped under the term engineered securement devices, as noted by the Infusion Nurses Society. (Tape and gauze aren’t included in this overview because they don’t provide adequate or appropriate securement.)
Sutures
Sutures are primarily a skin-closure solution, but they’ve been used as a securement option for I.V. lines and are considered the standard of practice for central venous catheter (CVC) securement. Sutures are frequently tied, often too tightly, close to the insertion site and catheter securement wings, creating multiple suture points. This method makes correct cleaning of the skin under the catheter difficult. Also, sutures create additional puncture sites in the skin, allowing bacteria to enter the subcutaneous surface via the suture material. This contaminated suture then resides under the dressing, which creates a moist environment for bacterial growth.
Adhesive securement devices
There devices, also known as engineered securement devices, were developed in response to the poor performance of sutures for CVC securement and to prevent accidental needlestick injury. Like sutures, these devices also can provide immediate securement. However, over time, adhesive degradation and loosening may occur. Strong adhesives that resist moisture require specific solvents for easy removal, and incorrect solvent use may result in skin damage. In addition, adhesives must be removed from the skin’s surface for proper cleaning of the insertion site and dressing area.
Subcutaneous securement device
Recently developed subcutaneous securement promotes stabilization and avoids pain receptors by securing the catheter to subcutaneous tissue at the insertion site, rather than the skin. Within 48 to 72 hours, the anchor heals into place, preventing catheter pistoning and side-to-side movement. The lack of movement promotes healing of the insertion site and allows the remodeled tissue to act as a barrier to surface bacteria. Because this securement option is stabilized in the puncture site, the VAD can be gently lifted above the insertion site, allowing for thorough cleaning.
Cyanoacrylate (tissue adhesive/“superglue”)
A novel approach to PIVC and CVAD securement is cyanoacrylate, a type of tissue adhesive or medical-grade “superglue,” which is typically used as an alternative to sutures for closing skin lacerations and repairing internal tissue. Tissue adhesive is a relatively new option for VAD securement, so little evidence exists to guide practice. However, growing evidence shows that glue may help prevent VAD-related infection by inhibiting Gram-positive organisms such as methicillin-resistant Staphylococcus aureus, a serious problem when isolated on vascular catheters. Manufacturers recommend using a solvent to remove the tissue adhesive, but Marsh and colleagues observed that some catheters came out easily without a solvent.
Integrated securement solutions
Integrated securement options represent an alternative to the application of two separate dressing and securement products (for example, suture and poly-urethane dressings). Newer-generation integrated products include reinforced fabric borders surrounding the polyurethane membrane, as well as additional adhesive components that hold the VAD from beneath.
A recent randomized control trial by Goossens and colleagues found a statistically significant reduced time for dressing changes (p < 0.001) when comparing adhesive and subcutaneous securement options. They also showed variable pain levels overall, and the usability of the adhesive was evaluated as statistically significantly more positive than a subcutaneous method at insertion and removal. These new technologies have the potential to reduce nursing procedural time.
Assessing outcomes
Bedside clinicians, who are responsible for providing care that achieves results that meet or surpass reimbursement outcome metrics (value-based purchasing), understand that different products influence patient outcomes. Outcomes also are influenced by overall clinician experience and knowledge of the available securement options and their use.
 Two studies illustrate how research can help clinicians make wise decisions when choosing a securement option.
Two studies illustrate how research can help clinicians make wise decisions when choosing a securement option.
A study by Marsh and colleagues highlighted that it took slightly longer to place a sutureless securement device, but the difference was only around 20 seconds— inconsequential compared to the time required for VAD replacement and the problems associated with VAD failure. This study also showed that tissue adhesive and other new PIVC securement products may considerably reduce failure. When converted into improvements in the patient experience and potential cost savings, this amount would be substantial.
A European study by Zerla and colleagues suggested that a subcutaneous securement device/product is highly efficient and cost-effective for securing medium (14 to 30 days) to long-term (more than 30 days) peripherally inserted central catheters (PICCs) with expected duration longer than 30 days. This engineered option had a positive impact in the researchers’ organization, reducing mechanical complications and the number of PICC replacements, a net decrease in the risk of therapy interruption and improved cost savings.
Preventing failure, reducing costs
Adequate securement of any VAD can help prevent mechanical and infectious complications. Using sutureless and engineered options provides a greater choice for optimizing VAD securement and, when used correctly and appropriately, minimizes potential risks of patient complications, with an overall cost savings and reduced needlestick exposure for all healthcare providers and patients.
Timothy R. Spencer is the director of Global Vascular Access, LLC, in Scottsdale, Arizona.
Selected Resources
Alexandrou E, Ray‐Barruel G, Carr PJ, et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med. 2015;10(8):530-3.
Corley A, Marsh N, Ullman AJ, Rickard CM. Tissue adhesive for vascular access devices: Who, what, where and when? Br J Nurs. 2017;26(19):S4-S17.
Goossens GA, Grumiaux N, Janssens C, et al. SecurAstaP trial: Securement with SecurAcath versus StatLock for peripherally inserted central catheters, a randomised open trial. BMJ Open. 2018;8(2):e016058.
Gorski L, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs. 2016;39(1S):S1-S169.
Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: Peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203.
Macklin D, Blackburn PL. Central venous catheter securement: Using the healthcare and technology synergy model to take a closer look. J Assoc Vasc Access. 2015;20(1):45-50.
Marsh N, Webster J, Flynn J, et al. Securement methods for peripheral venous catheters to prevent failure: A randomised controlled pilot trial. J Vasc Access. 2015;16(3):237-44.
Mattamal GJ. US FDA perspective on the regulations of medical-grade polymers: Cyanoacrylate polymer medical device tissue adhesives. Expert Rev Med Devices. 2008;5(1):41-9.
Rickard CM, Marsh N, Webster J, et al. Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (SAVE): A pragmatic, randomised controlled, superiority trial. Lancet. 2018;392(10145):419-30.
Rupp ME, Tandon H, Danielson P, Cavalieri J, Sayles H. Peripheral intravenous catheters—“They don’t get no respect.” Open Forum Infect Dis. 2017;4(suppl 1):S636.
Simonova G, Rickard CM, Dunster KR, Smyth DJ, McMillan D, Fraser JF. Cyanoacrylate tissue adhesives–effective securement technique for intravascular catheters: In vitro testing of safety and feasibility. Anaesth Intensive Care. 2012;40(3):460-6.
Timsit JF, Rupp M, Bouza E, et al. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med. 2018;44(6):742-59.
Ullman AJ, Cooke M, Rickard CM. Examining the role of securement and dressing products to prevent central venous access device failure: A narrative review. J Assoc Vasc Access. 2015;20(2):99-110.
Zerla PA, Canelli A, Cerne L, et al. Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access. 2017;18(3):238-42.
ant9-Vascular-827